|
Bumetanide (Bumex) is a diuretic drug (a medication that removes water, by increasing the production of urine). It is used to treat swelling caused by heart failure or liver or kidney disease. Recently, researchers in France have been exploring its use in Parkinson’s, and their results are really interesting. ‘Interesting’ because they not only point towards a clinically available drug that could (potentially) be repurposed for the treatments of Parkinson’s, but they also help to explain how our brains control movement. In today’s post we will review the new results, discuss what they suggest about our ability to move, and we will look at efforts to take this drug to the clinic for Parkinson’s. |
Source: Timemail
Heart failure (sometimes referred to as congestive heart failure) occurs when the heart is unable to pump sufficiently enough to maintain the required blood flow to meet the body’s needs. The most common causes of heart failure include coronary artery disease, high blood pressure, atrial fibrillation,valvular heart disease, and lifestyle issues (such as excess alcohol use). Overall around 2% of adults have heart failure; in those over the age of 65, this percentage increases to 6–10%. In 2015, it was estimated to affected approximately 40 million people worldwide (Source).
Common symptoms include:
- shortness of breath
- excessive tiredness
- leg swelling.
A common treatment option for heart failure are diuretics.
What are diuretics?
Diuretics (sometimes called water pills) are medications that have been designed to increase the amount of water and salt expelled from the body as urine.
There are three types of diuretic medications. They are:
- Thiazide
- Loop
- Potassium-sparing
Thiazide diuretics are the most commonly prescribed, generally for the treatment of high blood pressure. This class of drugs not only decreases the level of fluids in your body, they also cause your blood vessels to relax. Potassium-sparing diuretics reduce fluid levels in your body without – as the label suggests – causing you to lose potassium. The other types of diuretics can cause you to lose potassium, which can result in other health complications such as arrhythmia.
And then there are loop diuretics, which also decrease the level of fluid in the body.
But some loop diuretics have additional properties. And today we are going to have a look at one of them in the context of Parkinson’s.
It is called Bumetanide.
Why is Bumetanide interesting for Parkinson’s?
Recently an interesting study was published:

Title: GABAergic inhibition in dual-transmission cholinergic and GABAergic striatal interneurons is abolished in Parkinson disease
Authors: Lozovaya N, Eftekhari S, Cloarec R, Gouty-Colomer LA, Dufour A, Riffault B, Billon-Grand M, Pons-Bennaceur A, Oumar N, Burnashev N, Ben-Ari Y, Hammond C.
Journal: Nat Commun. 2018 Apr 12;9(1):1422.
PMID: 29651049 (This research article is OPEN ACCESS if you would like to read it)
In this study, the researchers found that half of the cholinergic interneurons in the striatum are both cholinergic and GABAergic. They also found that the dopamine depletion observed in Parkinson’s, results in these cholinergic/GABAergic interneurons becoming dysfunctional, and enhancing the inhibitory effect in the striatum. But more importantly, the investigators found that the loop diuretic Bumetanide rescued this effect.
Cool huh!
What does any of that actually mean?
Let’s start at the beginning.
The striatum is a structure deep in the brain. It is broken into two subregions in humans, called the putamen and the Caudate nucleus. The dopamine-producing neurons of the substantia nigra (these are the cells that are badly affected in Parkinson’s) have long projections (or axons) that extend up into the brain to the putamen and caudate nucleus, and this is where the cells release the bulk of their dopamine.
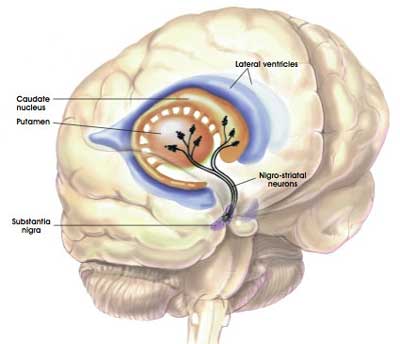
The projections of the substantia nigra dopamine neurons. Source: MyBrainNotes
The striatum is made up of different types of neurons.
The vast majority of the cells in the striatum are called Medium spiny neurons (also known as spiny projection neurons). They represent 95% of neurons within the human striatum, and they are a type of inhibitory cell. they produce a chemical called gamma–Aminobutyric acid (or just GABA).
GABA. Source: Wikipedia
GABA is a neurotransmitter. A neurotransmitter is a chemical that is used to pass a signal from one neuron to another. Dopamine – the chemical that is severely reduced in the Parkinsonian brain – is also a neurotransmitter.

A neurotransmitter being released by one cell (right) and binding to another. Source: Truelibido
Another population of cells in the striatum are the cholinergic interneurons. They make up only about 1–2% of all the cells in the striatum, but they are very large cells which send out dense networks of branches throughout the striatum, allowing them to communicate with lots of other cells. Cholinergic interneurons produce a neurotransmitter called acetylcholine.
Cholinergic neurons (green) and GABA neurons (red) in the striatum. Source: Neuro-marseille
Now the researchers in France who published the report we are reviewing today found that half of the cholinergic interneurons in the mouse striatum produce both acetylcholine and GABA. In the image below, note the white arrow pointing at the red cell (which is stained with a dye labelling the enzyme ChAT – which is required for the production of acetylcholine). That cell is also producing an enzyme called GAD 65 (white arrow in the middle panel), which is required for the production of GABA.
Example of a cholinergic GABA-producing interneuron. Source: Nature
The idea that neurons can produce and release two different neurotransmitters is not unique in the brain and it has been previously reported (Click here for an example). The researchers referred to these cholinergic GABA-producing interneurons in the striatum as CGINs.
When these CGIN neurons released the neurotransmitter acetylcholine, it was found to cause an excited response in neighbouring cells, while the GABA that was released was found to be inhibitory. The researchers suggested that these CGINs (or more importantly the balance between cholinergic and GABAergic activity from these cells) play a crucial role in the synchronisation of striatal networks, and they next wanted to look at what happens in the absence of dopamine – as in the case of Parkinson’s.
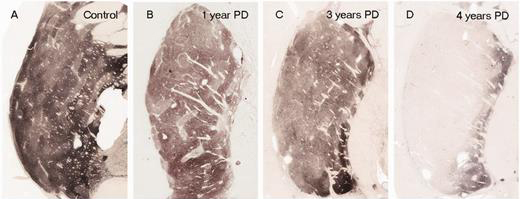
In Parkinson’s, the branches (or axons) of the dopamine neurons that extend up to the putamen and caudate nucleus gradually disappear as the dopamine neurons of the substantia nigra are lost. When one looks at brain sections of the putamen after the axons have been labelled with a dark staining technique, this reduction in axons is very apparent over time, especially when compared to a healthy control brain.
The putamen in Parkinson’s disease (across time). Source: Brain
EDITOR’S NOTE: I WOULD JUST LIKE TO ADD THAT THE IMAGE ABOVE IS NOT REPRESENTATIVE OF EVERYONE WITH PARKINSON’S. THE IMAGE IS BEING USED HERE TO PROVIDE AN EXAMPLE OF THE DOPAMINE FIBRE LOSS OBSERVED IN THE PUTAMEN. THIS PROCESS CAN TAKE LONGER IN SOME INDIVIDUALS THAN THE PERIOD OF TIME INDICATED.
In the absence of dopamine, the striatum gradually puts out a more and more inhibitory signal (blocking a person’s ability to move normally). This results in a very inhibitory signal being sent from the brain, down the spinal cord to the muscles of the body. So rather than having a carefully balanced combination of excitatory and inhibitory signals, the brain is sending out signals that inhibit more than encourage movement. Hence the slowness of movement witnessed in Parkinson’s.

Weakened excitatory signals (green) and enhanced inhibitory signals (red) in the Parkinsonian brain. Source: Animal Physiology 3rd Edition
So what happened to the CGIN neurons in the absence of dopamine?
The French researchers found that these CGIN neurons lost their ability to respond to GABA.
When the investigators treated the CGIN neurons of normal mice with a GABA receptor agonist – that is a molecule that stimulates the GABA receptor and inhibits cell activity (we have previously discussed agonists – click here to read more) – the CGIN cells activity reduced. But in mice that had been treated with a neurotoxin (6-OHDA) that killed the dopamine neurons, the GABA receptor agonist had no effect on the CGIN cells activity.
The CGIN neurons had lost their ability to respond to GABA.
This effect meant that the careful balance between cholinergic and GABAergic activity from these cells was completely thrown out of whack, leaving the CGIN cells continuously sending out an inhibitory signal. And this constant inhibitory signal, left the striatum even more inhibited.
If none of this is making any sense, understand one thing: the french researchers identified a drug that could correct this situation (both in cell culture and in mice).
Was that drug Bumetanide – the loop diuretic you mentioned above?
Yes, it was. Bumetanide.
But how does it fix the situation?
Bumetanide functions as an NKCC1 chloride importer antagonist.
And what on earth does that mean?!?
Ok, so chloride is an essential electrolyte.
And before you ask: An electrolyte is a substance that dissociates into ions in solution and acquires the capacity to conduct electricity. In neurons, this property is essential for maintaining cell homeostasis and transmitting action potentials in neurons.
An action potentials is a critical part of how one neuron passes a signal on to another neuron.
The action potential is an electrical impulse that is passed down the branch (or axon) of a neuron to the axon terminals where it stimulates the release of chemical messengers to pass the signal to the next neuron.
Source: KhanAcademy
When a neuron is at rest, there is a high concentration of sodium (Na+) ions and chloride (Cl-) ions outside of the neuron (in the extracellular fluid) compared the situation inside the cell (the intracellular fluid) where there is a high concentration of potassium (K+) ions.
Source: Washington
During an action potential, as the impulse is moving along the axon, this balance is reversed – sodium ions and chloride ions rush inside of the neuron, while potassium moves out. After the impulse has passed, there are active mechanisms that return the balance to normal (high concentration of sodium ions and chloride ions outside of the cell, etc).
This video will explain to you what an action potential is:
Now, an important feature of chloride is that while it is technically involved with action potentials, it is not actually essential for an action potential to occur.
Chloride can influence neuronal activity though. And adult neurons usually have low levels chloride, which makes them responsive to the actions of GABA. But when levels of chloride get too high, the neurons become unresponsive to GABA.
And this is exactly what the French researchers found: In the absence of dopamine, the CGIN neurons lost their ability to respond to GABA. And they discovered that this loss of response was due to very high levels of chloride in the cells.
So how does Bumetanide correct this?
Chloride is trafficked into and out of cells via chloride channels. NKCC1 is one of those channels.
NKCC1 aids in the active transport of chloride into a cell.
And Bumetanide is a NKCC1 chloride importer antagonist – that is a molecule that blocks the activity of NKCC1.
Source: Ahc
By inhibiting the NKCC1 chloride importer, Bumetanide efficiently restores low chloride levels in the neuron. This in turn returns the action of GABA to normal, which reduces the level of inhibition in the striatum.
And this effect was demonstrated behaviourally in mouse models of Parkinson’s.
The mice were administered the neurotoxin 6-OHDA 8 weeks before the behaviour testing, and they were treated daily with bumetanide (starting 3 weeks after the neurotoxin was given). These mice could perform the motor tests as well as normal control animals (with an intact dopamine system) and better than untreated mice (with dopamine loss).
To assess motor coordination, the mice firstly had to traverse a rolling beam and the time taken to do this was recorded (graph on the left below). The mice treated with the neurotoxin took longer to complete this task, compared with control animals and mice that were treated with the neurotoxin and Bumetanide. Similarly, in a second test of motor ability, the mice had to descend a vertical wooden pole (50 cm long and 1 cm diameter) leading to their home cage. Again the mice treated with the neurotoxin and Bumetanide performed better than their untreated counterparts (graph on the right below).
Bumetanide corrects behavioural deficits in mouse model of PD. Source: Nature
These results lead the researchers to conclude that high levels of chloride – causing constant GABA activity in CGINs – contribute to the motor problems produced by dopamine depletion. And this contribution can be reduced by lowering the levels of chloride in the cells via the treatment of the NKCC1 chloride importer, Bumetanide.
Interesting. Has Bumetanide ever been clinically tested in Parkinson’s?
No. But there has been a pilot study conducted by the same French research group that produced the study reviewed above. This is the research report:
Title: Bumetanide to Treat Parkinson Disease: A Report of 4 Cases
Authors: Damier P, Hammond C, Ben-Ari Y
Journal: Clin Neuropharmacol. 2016 Jan-Feb;39(1):57-9
PMID: 26757306
In this open label pilot study, the researchers treated 4 people with Parkinson’s with bumetanide daily for 2 months. They found that bumetanide was safe and well tolerated, and the treatment resulted in an improvement of Parkinson’s motor symptoms in all 4 subjects (as determined by the unified Parkinson’s disease rating scale (or UPDRS) both ON and OFF L-dopa medication). The investigators also reported that bumetanide improved gait and freezing in 2 of those participants.
Here is a table outlining the results in each case:
Source: Frontiersin
Based on all of these results, the researchers are now seeking to conduct a Phase II clinical trial to blindly evaluate bumetanide as a potential treatment for Parkinson’s in a cohort of 60 affected individuals.
They are hoping to do this via a company called B&A Therapeutics.
Source: B&A Therapeutics
So what does it all mean?
French researchers have proposed an interesting new approach towards treating Parkinson’s.
Usually, when someone is diagnosed, they are placed on dopamine-replacement therapies (such as L-dopa) which in many cases can restore normal motor function satisfactorily. Over time, however, higher and higher doses of L-dopa are required, which can result in undesirable complications (such as dyskinesias).
An alternative approach could be to simply reduce the level of inhibition in the motor regions of the brain. And this is what the French researchers have presented via the use of a heart failure medication. If successful in clinical trials, one would hope that such an approach would be complementary to L-dopa treatment, so that the dose of L-dopa an individual takes could be reduced and balanced out by the anti-inhibitory approach. This would reduce the amount of L-dopa an individuals needs to take, potentially slowing down the onset of dyskinesias.
I really like this idea and the possibility of offering people with Parkinson’s an alternative to just dopamine-based medication. But a key question that needs to be addressed in any clinical trials looking at bumetanide in Parkinson’s, is what are the consequences of long term use of this drug within this community. Is it really wise to start treating people with loop diuretics for long periods of time? This is particularly relevant because bumetanide is eliminated very rapidly in humans – it has a half-life of only 1 to 1½ hours (Source). If double-blind clinical trials do demonstrate a positive effect, future research could explore better targeting of the NKCC1 chloride importer.
While this approach may not be curative, it would offer the community yet another means of better managing this condition.
We will wait and watch with interest.
EDITOR’S NOTE: The information provided by the SoPD website is for information and educational purposes only. Under no circumstances should it ever be considered medical or actionable advice. It is provided by research scientists, not medical practitioners. Any actions taken – based on what has been read on the website – are the sole responsibility of the reader. Any actions being contemplated by readers should firstly be discussed with a qualified healthcare professional who is aware of your medical history. While some of the information discussed in this post may cause concern, please speak with your medical physician before attempting any change in an existing treatment regime.
The banner for today’s post was sourced from Medicinehow












Wonderful description! Thanks. Reminds me of the calcium channel blocker (currently in phase 3 testing), also for cardiac patients.
LikeLiked by 2 people
Thanks Diana, glad you liked the post. Have recently addressed calcium channel blockers and Parkinson’s in another post which may be of interest (https://scienceofparkinsons.com/2018/05/01/calcium/).
Kind regards,
Simon
LikeLike
thank you so much for this priceless for ordinary people information.
LikeLiked by 1 person
Hi Radosla,
You are very welcome. Glad you find the material interesting. Please let me know if there is a particular topic you would like to see discussed.
Kind regards,
Simon
LikeLiked by 1 person
Thank you for this interesting report. I am a renal physiologist and teach renal pharmacology at a medical school. I also have stage 3 Parkinson’s. I’m going to pass this on to some colleagues who specialize in the Na-K-Cl cotransporter to see what they think.
LikeLike
Could this be a way for patients whose Parkinsonism is not responsive to levodopa (e.g., due to a reduction in dopamine receptors) to attain a benefit that does not depend upon raising dopamine levels? For example, progressive supranuclear palsy is associated with a decrease in the number of dopamine receptors, and the value of levodopa therapy for PSP patients is therefore limited. Could bumetanide prevent chloride buildup inside the CGINs and thus allow PSP patients to regain some movement? Or if PSP also involves damage to the CGINs themselves, then are there perhaps other Parkinsonisms that could benefit from having an alternative to stimulating dopamine receptors?
LikeLike