Abstract
Pregnancy represents a dynamic period with physical and physiological changes in both the mother and her developing fetus. The dramatic 2–3 fold increase in the active hormone 1,25(OH)2D concentrations during the early weeks of pregnancy despite minimal increased calcium demands during that time of gestation and which are sustained throughout pregnancy in both the mother and fetus suggests an immunomodulatory role in preventing fetal rejection by the mother. While there have been numerous observational studies that support the premise of vitamin D's role in maintaining maternal and fetal well-being, until recently, there have been few randomized clinical trials with vitamin D supplementation. One has to exhibit caution, however, even with RCTs, whose results can be problematic when analyzed on an intent-to-treat basis and when there is high non-adherence to protocol (as if often the case), thereby diluting the potential good or harm of a given treatment at higher doses. As such, a biomarker of a drug or in this case “vitamin” or pre-prohormone is better served. For these reasons, the effect of vitamin D therapies using the biomarker circulating 25(OH)D is a far better indicator of true “effect.” When pregnancy outcomes are analyzed using the biomarker 25(OH)D instead of treatment dose, there are notable differences in maternal and fetal outcomes across diverse racial/ethnic groups, with improved health in those women who attain a circulating 25(OH)D concentration of at least 100 nmol·L−1 (40 ng·mL−1). Because an important issue is the timing or initiation of vitamin D treatment/supplementation, and given the potential effect of vitamin D on placental gene expression and its effects on inflammation within the placenta, it appears crucial to start vitamin D treatment before placentation (and trophoblast invasion); however, this question remains unanswered. Additional work is needed to decipher the vitamin D requirements of pregnant women and the optimal timing of supplementation, taking into account a variety of lifestyles, body types, baseline vitamin D status, and maternal and fetal vitamin D receptor (VDR) and vitamin D binding protein (VDBP) genotypes. Determining the role of vitamin D in nonclassical, immune pathways continues to be a challenge that once answered will substantiate recommendations and public health policies.
Similar content being viewed by others
Introduction
Pregnancy represents a time of immense change, which includes changes in physical proportions, physiology and responsibility. Arguably, nothing during these times changes more than the requirement and metabolism of vitamin D. Do the current recommendations for the requirements of vitamin D during these critical time periods reflect emerging data? Sadly, no. The minimal recommendations by the Institute of Medicine1 of 400–600 IU vitamin D per day and the 0 IU per day recommendation by The World Health Organization,2 was echoed by a recent Cochrane Review,3 stating that there are simply no requirements for vitamin D during pregnancy, are contrary to expanding published data (later reviewed in this chapter) that suggest otherwise. Why these recommendations continue to be made remains unclear, but clearly not recognizing a problem exists suggests that one does not need to address the “problem”.
During these dramatic times of physiological change, the roles of vitamin D in the pregnant versus, for example, the lactating woman are quite different. In the pregnant women, we believe the primary role of vitamin D to be an immunomodulatory—rather than a calcium-regulating factor, although, it would also retain that function. Further, vitamin D inadequacy in early life is clearly an instance of the “Barker Hypothesis”.4 This theory states that certain adult-onset diseases might have their roots in nutritional insults sustained in the perinatal period (either in utero or in the early months of infancy or both). Clearly, conditions associated with vitamin D deficiency such as asthma, multiple sclerosis (MS) and other neurological disorders would qualify.5,6,7,8,9,10,11,12,13,14,15
If one is reading this review for historical facts presented during the past 50 years of vitamin D recommendations during pregnancy, we suggest you read other recent publications for history in this area.16,17 However, if the reader is interested in gaining new insight into the vitamin D requirements and function during pregnancy supported by recent data, we suggest you read on. Also, as a word of caution, with regard to randomized controlled trials (RCT’s) for vitamin D, we discuss the criteria that must be asserted when applied to nutrient research, without which they are largely doomed to fail.18 The reasons for this are many and specific cases of this failure will be presented in this text.
Vitamin D nomenclature and metabolism
There are two forms of vitamin D: D2 and D3. Vitamin D2, or ergocalciferol, is made by plants, and fungi such as mushrooms; and vitamin D3, or cholecalciferol, is made by animals, including humans, and both are often referred to as the “parent compound”. For the remainder of the review, vitamin D will be used as a reference to both compounds unless otherwise noted.
Vitamin D3 is formed in the skin upon exposure to ultraviolet light exposure;19 vitamin D3 is also acquired through dietary supplementation along with vitamin D2 with the amount of this supplementation generally the source of lingering controversy.1,20,21,22 Since we are largely a society that avoids sun exposure, the role of dietary supplementation becomes extremely important. Once in the circulation, vitamin D is then converted into 25-hydroxyvitamin D [25(OH)D], which is the major circulating form of the vitamin. This conversion of vitamin D to 25(OH)D is achieved primarily in the liver but can also be achieved in a variety of tissues in an autocrine/paracrine fashion.23 Finally, 25(OH)D is converted into the hormonal form of the vitamin—1,25-dihydroxyvitamin D3 [1,25(OH)2D]—in the kidney for endocrine function and other tissues for autocrine/paracrine function.23 This concept is explained in detail elsewhere.23
Vitamin D metabolism during pregnancy when compared with the non-pregnant state
A striking difference exists in vitamin D metabolism during pregnancy and fetal development compared with non-pregnancy and non-fetal states, a point that has been known for at least the past three decades but which has received little attention until recently.24,25,26,27,28 The conversion of vitamin D to 25(OH)D appears unchanged during pregnancy, following first-and-zero-order enzyme kinetics.29 By contrast, the conversion of 25(OH)D to 1,25(OH)2D during pregnancy is unique and unparalleled during life. At no other time during life is 25(OH)D so closely linked with 1,25(OH)2D production. By 12 weeks of gestation, 1,25(OH)2D serum concentrations are more than twice that of a non-pregnant adult and continue to rise two- to threefold from the non-pregnant baseline rising to over 700 pmol·L−1, attaining levels that would be toxic due to hypercalcemia to the non-pregnant individual, but which are essential during pregnancy.30 Similarly, in the fetus, cord blood levels of circulating 1,25(OH)2D are even more closely tied to fetal levels of 25(OH)D.31,32 In neither the mother nor fetus does this conversion seem to be controlled by the classic calcium homeostatic mechanisms during the pregnant state.30,33
The rise in circulating 1,25(OH)2D levels in the mother/fetus is a remarkable observation. Early-on, the thought was that this increase was to ensure adequate delivery of calcium to the maternal skeleton preservation and fetal skeletal development. Calcium homeostasis, however, is not linked with this increase in 1,25(OH)2D, because at 12 weeks of gestation there is no increase in calcium demand by either the mother or fetus. In contrast, this increased concentration of 1,25(OH)2D sustained during pregnancy is not sustained during lactation when maternal calcium demand is at least as high as during pregnancy.34 Thus, in the mother and fetus during pregnancy, the rise in 1,25(OH)2D is dependent on substrate availability—in this case—25(OH)D, and is largely independent of calcium homeostasis.30
The control of circulating concentrations of vitamin D, 25(OH)D, and 1,25(OH)2D is a complex matter, affected by many disease states such as malabsorption syndromes, aberrant vitamin D metabolism as in sarcoidosis and/or disruptions in the calcium homeostatic system and so on.35 Although these are all important, they are beyond the scope of this short review and are not considered further; consideration is given only to what happens to these compounds under normal conditions when vitamin D is obtained through the diet or UV-light induction and how they enter normal cells.
In humans, vitamin D3 is naturally obtained when sunlight in the UVB range strikes the skin and causes 7-dehydrocholesterol to be converted, following a membrane-enhanced thermal dependent isomerization reaction, into vitamin D3, which then diffuses into the circulation through the capillary bed.36 Vitamin D also is obtained orally through the diet as either vitamin D2 or D3. As far as can be determined from the literature, this absorption process is primarily diffusion-based, is dependent on bile acid solubilization, and is not saturable.37,38,39 When vitamin D3 enters the circulation after UV exposure, it is primarily associated with vitamin D-binding protein (VDBP). In contrast, after intestinal absorption, it is coupled with both VDBP and lipoproteins.40 Vitamin D from either route is delivered primarily to the liver, where 25(OH)D is produced, becomes associated with VDBP, and is discharged into the circulation.41 Not only circulated to the liver, vitamin D also is circulated to all tissues in the body; many of which are now known to contain both the activating hydroxylase and the vitamin D 25-hydroxylase that converts vitamin D into 25(OH)D, thus achieving autocrine production of 25(OH)D in those tissues42,43,44,45,46 (Figure 1). We believe this to be an underappreciated and very important event that has not yet been adequately considered or investigated.
Diagram of the metabolic processes providing vitamin D and its metabolites to various tissues in the body. Tissue distribution of vitamin D and 25(OH)D based on simple diffusion (red arrows) or endocytosis (green arrows). Endocytosis requires the tissue-specific meglin-cubilin system, whereas simple diffusion is primarily controlled by the dissociation constant of the vitamin D compound for the VDBP. Bolder red lines indicate greater diffusion rates due to higher dissociation constant. t½, half-life.
On reaching the circulation, the primary determinant of how long a vitamin D metabolite will stay in circulation is its affinity for VDBP.47 Vitamin D, 25(OH)D and 1,25(OH)2D have vastly different dissociation constants with regard to VDBP: for 25(OH)D, it is ~10−9mol, and for vitamin D and 1,25(OH)2D, it is ~10−7 mol;48 in addition, for vitamin D, it is probably reduced to ~10−8 mol by its relative insolubility when measured in vitro.49 These dissociation constants also contribute to the circulating half-lives of these compounds, where for 25(OH)D, it is weeks; for vitamin D, 1 day; and for 1,25(OH)2D, a few hours.50–52 These dissociation constants also dictate the “free” concentration of compound that is available to enter into cells to be metabolized or to modulate cell activity (Figure 1). In the case of these three compounds, the “free” circulating concentrations are greater for 1,25(OH)2D than for intact vitamin D, which in turn is larger than that of 25(OH)D, matching their relative circulating half-lives.
Besides cellular diffusion of free compound, there exists another important tissue—the transport mechanism for these steroids-the megalin-cubilin endocytotic system.53 This system is key in the delivery of 25(OH)D to the 25-hydroxyvitamin D-1-α-hydroxylase in the kidney,54 which also exists in the parathyroid glands, making its important role in the endocrine function of vitamin D self-evident.55 The megalin-cubilin system also functions in the placenta56 and brain,57 which we will revisit later. Where tissues lack this endocytotic system, however, diffusion of vitamin D compounds in relation to free circulating concentrations becomes inherently important. Interestingly, VDBP-knockout animal models show normal survival when given dietary vitamin D on a daily basis.58,59 Because vitamin D metabolite cellular access could only be by diffusion in these animals, this shows that the parent compound vitamin D is normally transferred in wild-type animals through simple membrane diffusion.
Why is calcium metabolism uncoupled from 1,25(OH)2D generation during pregnancy and not lactation? One of the leading theories is that 1,25(OH)2D is an important immune modulator involved in maternal tolerance to the foreign fetus whose DNA is only half that of the mother’s. Early epidemiological studies involving pregnant women with preeclampsia, a clinical picture of inflammation and vasculitis, vitamin D deficiency has been implicated.60,61 Experimental animal models have also strongly suggested vitamin D deficiency as a potential mechanism of placental dysfunction62,63 and respiratory maturation.64
Vitamin D is a known modulator of inflammation.65 Native dietary vitamin D3 is thought to be bio-inactive, and the beneficial effects of vitamin D are thought to be largely mediated by 1,25(OH)2D.23 In many disease states, low circulating 25(OH)D are associated with multiple inflammatory diseases, such as cardiovascular, arthritis, MS, cancer and sepsis.5,6,7,8,9,10,11,12,14,15,66 Common to all of these diseases is the disruption of endothelial stability and an enhancement of “vascular leak.” Experimental animal models of preeclampsia clearly demonstrate this endothelial instability leads to placental ischemia.67 To that end, Gibson et al.68,69 have identified vitamin D3 as a very effective stabilizer of endothelium and endothelium “leak” through non-genomic mechanisms. This membrane stabilization function is highly structurally specific in that an open b-ring, the cis-triene structure of vitamin D, is required.68 This is an incredible new observation. What these studies demonstrate is that vitamin D3, 25(OH)D3 and 1,25(OH)2D3 all have the ability to control “endothelial leak”. Most surprising is that on an equal molar basis, vitamin D3 is more potent in this function than are 25(OH)D3 or 1,25(OH)2D.68
As shown in Figure 1, this observation is taken one step further. Besides being the most potent stabilizer, vitamin D3 would also be the metabolite most accessible to the cell membrane to impart that function. That is because circulating 25(OH)D3 is almost totally bound to the VDBP and its “free” concentration so miniscule that there simply is not enough to matter. 1,25(OH)2D3, while existing in a high circulating “free” form, simply circulates at a level of insignificance for this function. While vitamin D3 following its synthesis in the skin would have a slightly longer half-life as opposed to oral dose vitamin D, these differences are minor compared to the half-life of 25(OH)D, which is weeks compared to days.40 Given the perceived risks of UV-light exposure, dosing currently in vitamin D studies is by oral supplementation rather than UV-light therapy. Vitamin D3, then, if given at physiological doses of 4 000 IU·d−1 or greater, would circulate in the “free” form at significant levels and be available to membrane insertion and subsequent endothelial stabilization that is likely to have profound effects on several disease processes. This is truly a new frontier in vitamin D mode of action.
Obstetrical “paranoia” with regard to vitamin D administration during pregnancy
We refer to this type of thinking as “medical lore”; however, in this particular case because it carries forth into current medical care, we view it as dangerous. It happens when medical students are taught something that is based on obsolete data that have carried through to the present. This is absolutely the case with the use of vitamin D during pregnancy. Why is this?
Because of the British experience with idiopathic infantile hypercalcemia attributed to hypervitaminosis D, a terrible, inaccurate association occurred that had a profound effect on the potential of vitamin D supplementation, not only during infancy but also during pregnancy. In 1963, Black and Bonham-Carter70 recognized that elfin facies observed in patients with severe idiopathic infantile hypercalcemia resembled the peculiar facies observed in patients with supravalvular aortic stenosis (SAS) syndrome. Shortly thereafter, Garcia et al.71 documented the occurrence of idiopathic hypercalcemia in an infant with SAS who also had peripheral pulmonary stenosis, mental retardation, elfin facies and an elevated blood concentration of vitamin D. This is an interesting observation because, in 1964, when the article was published, there were no quantitative means of assessing circulating concentrations of vitamin D. In fact, at that time, it was not even proven that vitamin D was further metabolized within the body. By 1967, vitamin D was viewed by the medical community as the cause of SAS syndrome.72,73 As a result of the theory that maternal vitamin D supplementation during pregnancy caused SAS syndrome,74 animal models were developed to show that toxic excesses of vitamin D during pregnancy would result in SAS.75,76 In these earlier cases,70 vitamin D had nothing to do with the etiology of SAS. What was described as vitamin D-induced SAS syndrome is now known as Williams Syndrome.77,78 Unfortunately, vitamin D intake during pregnancy is still associated with SAS.
Williams Syndrome is a rare developmental disorder and severe genetic affliction related to elastin gene disruption77 that is caused by 26–28 elastin and contiguous deleted genes on chromosome 7 g 11.23. This syndrome is characterized by multiorgan involvement (including SAS), dysmorphic facial features, and a distinctive cognitive profile.78 Such patients often exhibit abnormal vitamin D metabolism, which makes them susceptible to bouts of idiopathic hypercalcemia.79 This relationship was suspected as early as 1976 by Becroft and Chambers.80 Subsequently, Taylor et al.81 demonstrated that children with Williams Syndrome exhibit an exaggerated response of circulating 25(OH)D to orally administered vitamin D. Thus, the fear of vitamin D-induced SAS is based on studies that are no longer valid yet continue to be cited, feared and thus impact treatment.
Observational studies suggesting the function of vitamin D extended beyond calcium homeostasis during pregnancy
Again, this review will not discuss the role of vitamin D and skeletal function during pregnancy since this has been discussed endlessly in the past, and truth be told, minimal supplemental vitamin D is required to meet these needs.1,16,17,82 Certainly, these needs would be met and exceeded by recommendations we make here with respect to non-skeletal functions of vitamin D.
Beyond skeletal issues, what would these other issues be with respect to vitamin D in pregnancy? To discover what these might be, we rely on associative or observational studies, and in the past 15 years or so many of these studies have been performed. Of high interest is the association of dietary vitamin D3 intakes in pregnant women and preeclampsia. Olsen and Secher83 point out that in the early 1940s, studies were performed giving pregnant women halibut liver oil, a rich source of vitamin D3, with decreases in preterm birth and preeclampsia observed, which the authors attributed to marine n-3 fatty acids, with no mention of vitamin D and its potential effect.83 But why would they, since that connection would make no sense to them at the time? As we moved through the recent decades, it became clear that vitamin D and its metabolites’actions in the human body could exist well beyond skeletal events, and thus people started looking at the link between vitamin D and other disease states and conditions.5,6,7, 8,9,10,11,12,13,14,15,60,61,84,85,86,,87,88,89Early observational studies uncovered strong relationships between maternal circulating levels of 25(OH)D and preeclampsia,60,61,84,85 altered placental vascular pathology,86 cesarean section,87 glucose tolerance,88 adverse birth outcomes due to race,89 infection rates,5 brain function,13,14,15 and respiratory function.6 More recent studies have pointed to maternal vitamin D deficiency as a risk factor for abnormal fetal growth patterns, adverse birth outcomes, and reproductive failure.90,91,92,93,94 Also, a recent meta-analysis of observational studies has confirmed the fact that maternal vitamin D deficiency increases the risk of preterm birth.95
Although public policy cannot be set for supplementation practices based on observational studies, this information is invaluable at pointing research in the direction that could yield public policy changes in vitamin D consumption. These next steps are interventional studies or better yet, randomized clinical trials (RCTs). One has to exhibit caution, however, even with RCTs, whose results can be problematic when analyzed on an intent-to-treat basis and when there is high nonadherence to protocol (as is often the case), thereby diluting the potential good or harm of a given treatment at higher doses. As such, a biomarker of a drug or in this case “vitamin” or preprohormone is better served. For these reasons, analyses of effect of vitamin D therapies using 25(OH)D concentration is a far better indicator of true “effect”.
Randomized controlled trials investigating vitamin D supplementation during pregnancy
Enthusiasm for evidence-based medicine (EBM) has resulted in the extension of its methods to the evaluation of nutrient effects. Heaney18 has pointed out EMB, as applied in the evaluation of drugs, is poorly suited to the study of nutrients. In a drug trial, the placebo group will be totally devoid of the compound in question; not so for a nutrient like vitamin D. To perform a true RCT for vitamin D one would have to make sure all subjects were vitamin D-deficient at the study onset. For the duration of the study all subjects would have to remain indoors to avoid any sun exposure. Then and only then could a true RCT be performed for any given function of vitamin D. Of course, what we have suggested here is unethical and is never going to take place. How then does one proceed? Heaney18 has provided excellent guidance in this regard. Dr. Heaney proposes five rules for individual clinical studies of nutrient effects. These rules are as follows: (1) basal nutrient status must be measured, used as an inclusion criterion for entry into the study, and recorded in the report of the trial; (2) the intervention must be large enough to change nutrient status and must be quantified by suitable analysis; (3) the change in nutrient status produced in those enrolled in the report of the trial must be measured and reported; (4) the hypothesis to be tested must be that a change in nutrient status produces the sought-for-effect; and (5) co-nutrient status must be optimized in order to ensure that the nutrient is the only nutrition-related, limiting factor in the response. We would add one additional rule to this group, that being: the nutrient in question has to follow an appropriate dosing schedule matching the physiological system being investigated.23 Needless to say, while almost all vitamin D RCT’s to this point would fail based on these criteria, evaluation of existing evidence with respect to pregnancy will become the basis for optimizing dietary and clinical recommendations.
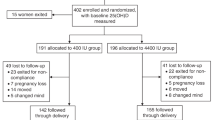
Vitamin D supplementation trials involving pregnant women have been performed since 1980.96 These early studies were small, did not look at meaningful end points such as accurate bone mineral density measurements (such methods did not exist at that time), or the role of vitamin D on the incidence of such disease states as preeclampsia,61,84,85,97 asthma,98–102 preterm birth,95,103,104 and autoimmune dysfunction,105–109 and/or supplemented with nominal doses of vitamin D (0–400 IU·d−1).96 As a result, no meaningful information or public policy changes occurred because of them. In 2001, our group conceived a large RCT investigating the supplementation of vitamin D to a pregnant population. Our study was radical in design in that we proposed supplementing pregnant women less than 16 weeks of gestation with up to 4 000 IU·d−1 vitamin D3 until delivery in a double-blind fashion. The goal of the study was to see how much vitamin D was required to raise circulating maternal 25(OH)D concentrations to at least 32 ng·mL−1 by the end of gestation. Using mathematical calculations from previous studies, we calculated how much vitamin D3 we would need to provide to achieve this endpoint.110,111 We selected the 32 ng·mL−1 concentration of circulating 25(OH)D based on the suppression of secondary hyperparathyroidism.112 We obtained funding from the National Institute of Child Health and Development in 2002; however, because of concerns about the safety of our 4 000 IU·d−1 dose of vitamin D3, we had to obtain an investigational new drug application approval from the Food and Drug Administration (FDA; #66 346). This approval was obtained in 2003 and the study began in early 2004. Along with this National Institute of Child Health and Development-sponsored study, we also received funding from the Thrasher Fund to perform a parallel study involving vitamin D supplementation of pregnant women in a community-based format. At the initiation of these RCTs, our end points were safety of the dosing, attained circulating level of maternal 25(OH)D, growth parameters of the infant, and bone-mineral-density of the mother and infant. As for the other factors mentioned in the previous section on vitamin D relationships based on observational studies, those associations had not been made at study initiation, and as a result we had no idea to even look for them, let alone propose them as end points. As such, these end points were analyzed as post hoc analyses.
The results of these RCT’s have been presented and published over the past few years.30,99,104,113–121 The main finding of these studies was that a 4000 IU·d−1 dose of vitamin D3 safely elevates circulating 25(OH)D to a level that, regardless of race, fully normalizes vitamin D metabolism and calcium homeostasis in the pregnant women. Using repeated measures, the concentration of 25(OH)D that fully normalized 1,25(OH)2D in our study cohort was determined on each subject and plotted to determine the point at which first order kinetics went to zero order.30 This point was determined to be when the 25(OH)D concentration was 40 ng·mL−1, the production of 1,25(OH)2D became substrate independent.30 Further, this dose was safe with not a single adverse event observed attributable to vitamin D supplementation.30,99,104, 113–121
When our studies were completed in 2009–2010, we were aware of the observational data suggesting favorable efforts of vitamin D on pregnancy outcomes beyond calcium homeostasis. Of course we had collected all of the data on our patient’s outcomes during the trials for safety reasons, and so they were subsequently analyzed. These data were first presented in 2009 at The Vitamin D Workshop in Brugge, Belgium. The data from our studies, when analyzed on an intent-to-treat basis, clearly demonstrated increased vitamin D supplementation decreased complications of pregnancy and C-section births.30,113 Further, RCT data and analysis by our group and others have clearly demonstrated that higher doses of vitamin D during pregnancy improve birth outcome data.104,113 RCT studies beyond our own have recently demonstrated vitamin D to greatly decrease complications of birth and gestational diabetes,116,117,120 aeroallergen sensitization119 and markers of regulatory immunity.121 The most informative of these RCT studies was performed by Sablok et al.116 These investigators took a vitamin D-deficient population of pregnant women, with circulating 25(OH)D concentrations of <10 ng·mL−1, and supplemented the treatment arm with substantial amounts of vitamin D starting at 20 weeks of gestation. The control group received placebo and thus remained profoundly vitamin D deficient throughout pregnancy. Vitamin D treatment in these women resulted in a substantial decline in the complications of pregnancy (Figure 2). Further, the compliance rate of the women was 100% because the physicians administered the vitamin D to each patient. A study of this type could never be performed in the US as it would be deemed unethical. Be that as it may, it demonstrated the highly significant effect of vitamin D supplementation on the complications of pregnancy. These findings were novel and controversial when they were first presented in 2009 in Belgium.Despite there being high evidence (level 1B) for changing vitamin D dosing and definitions of sufficiency during pregnancy, there was much resistance to change; however, with time and supportive data,these “new” conceptshave prevailed above bias.
Effect of vitamin D supplementation starting at 20 weeks of pregnancy with respect to the development of complications of pregnancy. Pregnancy complication in the form of preterm labor (PTL), gestational hypertension (GHTN)/preeclampsia (PE) or gestational diabetes mellitus (GDM) were observed in 25/57 (44%) women taking placebo compared to 22/108 (20.4%) women being supplemented with vitamin D. Significance between groups was P<0.02. Reproduced with permission from Sablok et al.116
Supplementing vitamin D during pregnancy to prevent childhood asthma
In 2006, Dr Hollis was contacted by Scott Weiss, MD of the Harvard Medical School with an idea to conduct a RCT using vitamin D supplementation during pregnancy to prevent the development of childhood asthma. Dr Weiss was aware of our ongoing RCT and had excellent observational data suggesting vitamin D supplementation during pregnancy could reduce childhood asthma rates.122,123 Subsequently, we obtained funding for this project from The National Institute of Heart, Lung and Blood (NHLBI) and the Vitamin D Antenatal Asthma Reduction Trial (VDAART) was born. In a collaboration between Boston University, Brigham and Women’s Hospital, Harvard Medical School, Kaiser Permanente South California Region, Medical University of South Carolina, Washington University in St. Louis, and NHLBI, a double-blind RCT was performed at three clinical centers: Boston, St. Louis and San Diego; and involved giving supplemental vitamin D3 (400 or 4 400 IU·d−1) to pregnant women across the three major racial/ethnic groups in the US from 16 weeks of gestation until delivery. The primary endpoint was prevention of asthma/wheeze in the infant/child at 1–3 years post birth. Nearly 900 high-risk subjects were enrolled and completed the study, which recently was published.99 The results of this study are quite clear: vitamin D supplementation during pregnancy will decrease asthma or recurrent wheezing rates in children (Figure 3).
Kaplan–Meier survival estimates for the effect of vitamin D treatment during pregnancy on the development of asthma/recurrent wheeze by age 3 year analyzed in an intent-to-treat format. The hazard ratio for the time to first event of asthma or recurrent wheeze was 0.8 at 3 years, P=0.051. Reproduced with permission from Litonjua et al.99
A nearly identical RCT study performed in Denmark also recently has been published.118 The journal in which these articles appeared—JAMA-has attempted to minimize the results and impact of these studies with an Editorial.124 In response to this negativity, the authors of these two RCTs performed a meta-analysis (H Wolsk, personal communication). Keep in mind, that a meta-analysis of RCTs is the highest form of validation for therapy/prevention/etiology/harm as defined by The Centre for Evidence-Based Medicine at Oxford University.125 The results from these RCTs and meta-analysis studies in women with offspring at high risk for asthma are quite clear: vitamin D3 given to a pregnant woman will prevent asthma/wheeze in her child (H Wolsk, personal communication).99,118 The mechanisms by which this occurs remain unknown but it is likely that epigenetic in utero changes triggered by vitamin D administered to the pregnant women impart functional changes in the fetus.126–128
Further analysis of the VDAART study published in JAMA99 reveal some startling findings. Included in that publication “buried” in the supplemental data is the following: Weiss et al have analyzed the data by post hoc stratification by maternal third trimester circulating 25(OH)D levels. In this case, circulating 25(OH)D serves as a biomarker of patient compliance for taking supplemental vitamin D during pregnancy. In the JAMA publication, adherence or compliance was a huge problem that could not be dealt with in the intent-to-treat study analysis.99 This non-adherence was especially acute in the African-American subjects who comprised 43% of the total study subjects and adhered to the prescribed supplementation regimen—as assessed by pill counts and electronic medical cap monitoring—50% of the time. What was the result from this? This non-adherence could bias the study toward null results. While analyses that take into account compliance may have some intrinsic bias as behaviors for taking the study pill could be associated with other behaviors that affect the outcome, nonadherence can significantly affect results of clinical trials, especially in the higher-dose treatment groups where nonadherence can dilute the treatment effect. In these trials, when this bias is factored into the results, the strength of the findings becomes more significant.98,99 If one uses circulating 25(OH)D levels as a biomarker of adherence to protocol, the effect of vitamin D preventing childhood asthma becomes highly significant (P<0.02; Figure 4).98,100 This effect is especially true for the African-American pregnant women in our VDAART study.101
Kaplan–Meier survival estimates for the effect of vitamin D treatment during pregnancy on the development of asthma/recurrent wheeze by age 3 year analyzed stratified by third trimester maternal level of circulating 25(OH)D as an estimate of study compliance. The hazard ratio for the time to first event of asthma or recurrent wheeze now becomes 0.73 at 3 years, P<0.02. Reproduced with permission from Litonjua et al.99
Vitamin D-induced genomic alterations during pregnancy
If one looks at our original pregnancy study from an intent-to-treat fashion, the results are muddled most likely due to nonadherence;30 however, taking adherence into account by using circulating 25(OH)D levels as a variable, the true effect on vitamin D supplementation on preterm birth is exposed104,114 (Figures 5 and 6). The same associations from our VDAART trial also hold true for the prevention of preeclampsia.129 How does this occur? Vitamin D supplementation during pregnancy appears to affect genetic information of several highly functional modules related to systemic inflammation and immune responses and implicates the emergence of a distinctive immune response in women destined to develop preeclampsia.127,130
Circulating levels of maternal 25(OH)D with respect to birth staging. Reproduced with permisson from Wagner et al.104
LOESS curve of 25(OH)D concentration and gestational age (weeks) at birth to show the change in average behavior with 1 and 2 s.d. windows superimposed (NICHD and TRF, n=509). Black line represents fitted LOESS curve; dark gray area represents 1 s.d.; and light gray area represents 2 s.d. Multivariable log-binomial regression found that 25(OH)D concentrations >40 ng·mL−1 reduces the risk of preterm birth by 59% compared to <20.0 ng·mL−1, adjusted for covariates. NICHD, National Institute of Child Health and Development. Reproduced with permission from Wagner et al.104
A recent paper by Al-Garawi et al,128 derived from the VDAART RCT study, provides direct proof of vitamin D’s ability to induce genomic changes during pregnancy. Patterns of gene expression during human pregnancy are poorly understood. As mentioned earlier, this study was a RCT of vitamin D supplementation in pregnancy for the reduction of pediatric asthma risk.94 The trial enrolled 881 women at 10–18 weeks of gestation. Longitudinal gene expression measures were obtained on 30 pregnant women, using RNA isolated from peripheral blood samples obtained in the first and third trimesters. Differentially expressed genes were identified using significance of analysis of microarrays (SAM) and clustered using a weighted gene co-expression network analysis (WGCNA). Gene-set enrichment performed to identify major biological transcriptional profiles between first and third trimesters of pregnancy identified 5 839 significantly differentially expressed genes.Weighted gene co-expression network analysis clustered these transcripts into 14 co-expression modules of which two showed significant correlation with maternal vitamin D levels (Table 1). Pathway analysis of these two modules revealed genes enriched in immune defense pathways and extracellular matrix reorganization as well as genes enriched in notch signaling and transcription factor networks (Table 2 and Figure 7,Figure 8). These data suggest that maternal gene expression changes during pregnancy and that these changes are related to vitamin D supplementation that increase circulating vitamin D levels. What remains unclear is whether these changes in maternal vitamin D levels impact fetal development directly or whether there is any direct effect of maternal gene expression on the fetus.128 Another question is whether or not the effects of vitamin D supplementation on gene expression observed in the maternal circulation differ from those effects present in the fetal and maternal placental microenvironment. In addition, the peripheral mononuclear cells themselves are a mixed population with vastly different circulating half-lives that likely have differentially expressed maternal genes. Such factors and issues suggest the need for continued investigation to decipher vitamin D and its metabolites’ dynamic effects on genetic and epigenetic expression during pregnancy that encompasses both the mother and the developing fetus.
Weighted Co-expression Network Analysis (WGCNA) was carried out on 5839 differentially expressed probes identified by SigGenes. 14 co-expression modules were identified and, correlated to various clinical traits. Gene network, represented by different colored coded co-expression modules (y axis) and their association with various clinical traits (x axis). The intensity of the colors indicates the strength of the relationships, as indicated by the scale to the right. The range of the scale (+1 to −1) indicates either positive (+1) or negative (−1) correlation with a specific clinical trait. Top number in each box corresponds to the Pearson’s correlation coefficient between a module and a specific trait, while the lower number represents its P-value. Traits: ppbmi=pre-pregnancy BMI; gestdays=gestational age; basev=vitamin D levels in first trimester; latev=vitamin D levels in third trimester; mother.race=maternal race (White/African-American); Child.gender+infant gender (boy/girl). Pearson’s correlation (P<0.05). Reproduced with permission from Al-Garawi et al.128
CREB1 transcription factor network depicting CREB1 in center and known interactions among 72 genes demonstrating various functionality within the green module. Hypergeometric test adjusted for multiple comparisons using Benjamini and Hochberg (P<0.05). Reproduced with permission from Al-Garawi et al.128
Maternal vitamin D supplementation also is involved in epigenetic regulation in children. Pathways affected by DNA methylation, for example, include antigen processing and presentation, inflammation, regulation of cell death, cell proliferation, transmission of nerve impulse, neurogenesis, neuron differentiation, sensory organ development126 and vitamin D metabolism.131 These modifiable effects of vitamin D on gene expression are truly impressive by any standard.
What is clear from these recent RCTs is that a 4 000 IU·d−1 vitamin D3 supplement is beneficial to both mother and child and these benefits have nothing to do with the classic role of vitamin D in calcium homeostasis. In fact, maternal hypercalcemia attributed to placental overproduction of 1,25(OH)2D has never been reported30,99 and the hypercalcemia found in women with molar pregnancies is attributable to PTHrp overproduction.132,133 What is not resolved is the dose and time of administration to achieve optimum results. We believe that a target circulating 25(OH)D concentration of 40 ng·mL−1 be achieved in pregnancy as early as possible. Because of biochemical heterogeneity in attaining a given concentration of 25(OH)D, we believe all womenshould consume at least 4 000 IU·d−1 vitamin D3 before conception.111
Neurodevelopment and autoimmune consequences
Can vitamin D deficiency and subsequent vitamin D supplementation during pregnancy impact autoimmune disease and neuropsychological development? There are some compelling data that suggest an association. It has long been thought that the development of MS is a result of a complex interaction between genes and environment with an important environmental factor being vitamin D deficiency.11 It is not yet understood how and when vitamin D acts to modulate MS risk, although there is increasing evidence that this occurs through genetic alterations.10
Data have emerged that demonstrate that vitamin D supplementation during pregnancy alters transcriptome and epigenetic alterations through DNA methylation in genes that regulate metabolic processes, antigen processing, inflammation, regulation of cell death, cell proliferation, transmission of nerve impulse, neurogenesis, neuron differentiation and sensory organ development.126 For now, what we have are observational studies strongly suggesting vitamin D deficiency during pregnancy as a strong causative agent in the development of MS in later life.7,9 Agencies such as the Institute of Medicine and Centers of Disease Control will say that this data must be confirmed by RCT. We state here that such an RCT will NEVER be performed because of the cost involved. How do we know that? A few years ago MS world experts were assembled by the National Multiple Sclerosis Society in Chicago to help design such a study. Following two days of meetings, it was determined that the minimum dollar amount to conduct such an RCT would exceed 50 million dollars and would consume their entire budget for at least 5 years. While the study never went forth and never will, other RCTs of treatment to prevent progression of disease for example, may be conducted. Further, an article by Mokry et al on Mendelian randomization provides strong support of a causal association of vitamin D and lower MS risk in humans.134 Health providers will have to make decisions on the data that are available that come from corollary studies such as these.
An even scarier prospect exists around vitamin D deficiency during pregnancy and neurological disease and altered development.12–15,135,136,137 Strong experimental animal evidence points to dire neurological consequences if vitamin D is restricted during pregnancy.15 If one wants to read the biochemical basis for this we suggest you read recent reviews by Patrick and Ames.138,139 They make an excellent case for intrauterine vitamin D deficiency as it relates to autism, attention deficit disorder, bipolar disorder, schizophrenia and impulse behavior all through the control of serotonin synthesis in the neonatal brain.138 There is also a fair amount of observational data available to support these claims.12–15 If that is not convincing we suggest you read a recent prospective, interventional vitamin D trial during pregnancy for the prevention of autism in the newborn.12 From this data the authors suggest that even performing an RCT would be unethical.12
Current recommendation for vitamin D supplementation during pregnancy
At this time, based on RCT data as well as substantial observational and interventional data, we suggest that all pregnant women maintain a circulating 25(OH)D concentration of at least 40 ng·mL−1 during the earliest time points of pregnancy.104 This will insure maximum protection from pregnancy complications, including preeclampsia in the mother and asthma formation in the infant. To achieve this, intakes of at least 4 000 IU·d−1 vitamin D3 will be required because of variable individual abilities to convert vitamin D to 25(OH)D.111 These supplements have proven to be safe in thousands of patients over the past 15 years, as not a single adverse event due to supplementation has been observed. Further, this level of supplementation lies within the safe intake level as defined by The Endocrine Society.22 Finally, does vitamin D qualify as a substance as described by the Barker Hypothesis? The clear answer is yes; it does because its absence during pregnancy imparts detrimental genetic alterations on both mother and fetus.
Summary
At no other time during the lifespan is vitamin D status more important than during pregnancy, affecting not only the mother but also her growing fetus, and later, her growing infant. While there has been considerable controversy surrounding the daily requirement of vitamin D and what constitutes sufficiency during these critical periods, there is mounting evidence of the importance of vitamin D supplementation during pregnancy to achieve a total circulating 25(OH)D concentration of at least 40 ng·mL−1, the point at which the conversion of 25(OH)D to 1,25(OH)2D is optimized and associated with a lower risk of comorbidities of pregnancy and better outcomes. Past data suggesting that vitamin D is a teratogenic compound is completely unfounded at the physiological doses reviewed in this chapter. As has been shown, significant amounts of vitamin D—whether their source is sunlight or supplement—are required during pregnancy to protect the mother and fetus and impart genomic imprinting on the fetus to ensure long term health.
With enhanced knowledge about vitamin D’s role as a preprohormone, it is clear that recommendations about supplementation must mirror what is clinically relevant and evidence-based. Future research that focuses on the critical period(s) leading up to conception and during pregnancy to correct deficiency or maintain optimal vitamin D status remains to be studied. In addition, what effects vitamin D has on genetic signatures that minimize the risk to the mother and developing fetus have not been elucidated. Clearly, while there is much more research that needs to be performed, our understanding of vitamin D requirements during pregnancy has advanced significantly during the past few decades.
References
Ross AC, Manson JE, Abrams SA et al. The 2011 Dietary Reference Intakes for Calcium and Vitamin D: what dietetics practitioners need to know. J Am Diet Assoc 2011; 111: 524–527.
World Health Organisation. Vitamin D Supplementation in Infants. Geneva: World Health Organisation. 2014.
De-Regil LM, Palacios C, Ansary A et al.Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev 2012; 2: CD00887.
Heaney RP . Is vitamin D inadequacy in early life an instance of the "Barker Hypothesis"? Nutr Today 2016; 51: 14–17.
Camargo CA Jr, Ingham T, Wickens K et al. Cord-Blood 25-Hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthama. Pediatrics 2011; 127: e180–e187.
Litonjua AA . Childhood asthma may be a consequence of vitamin D deficiency. Curr Opin Allergy Clin Immunol 2009; 9: 202–207.
Munger KL, Aivo J, Hongell K et al. Vitamin D status during pregnancy and risk of multiple sclerosis in offspring of women in the Finnish Maternity Cohort. JAMA Neurol 2016; 73: 515–519.
Greenberg BM . Vitamin D during pregnancy and multiple sclerosis: an evolving association. JAMA Neurol 2016; 73: 498–499.
Dobson R, Giovannoni G, Ramagopalan S . The month of birth effect in multiple sclerosis: systematic review, meta-analysis and effect of latitude. J Neurol Neurosurg Psychiatry 2013; 84: 427–432.
Berlanga-Taylor AJ, Disanto G, Ebers GC et al. Vitamin D-gene interactions in multiple sclerosis. J Neurol Sci 2011; 311: 32–36.
Ebers G . Interactions of environment and genes in multiple sclerosis. J Neurol Sci 2013; 334: 161–163.
Stubbs G, Henley K, Green J . Autism: Will vitamin D supplementation during pregnancy and early childhood reduce the recurrence rate of autism in newborn siblings? Med Hypotheses 2016; 88: 74–78.
McGrath JJ, Eyles DW, Pedersen CB et al. Neonatal vitamin D status and risk of schizophrenia: a population-based case-control study. Arch Gen Psychiatry 2010; 67: 889–894.
Cannell JJ . Autism and vitamin D. Med Hypotheses 2008; 70: 750–759.
McGrath JJ, Féron FP, Burne TH et al. Vitamin D3-implications for brain development. J Steroid Biochem Mol Biol 2004; 89-90: 557–560.
Brannon PM, Picciano MF . Vitamin D in pregnancy and lactation in humans. Annu Rev Nutr 2011; 31: 89–115.
Abrams SA . Vitamin D supplementation during pregnancy. J Bone Miner Res 2011; 26: 2338–2340.
Heaney RP . Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr Rev 2014; 72: 48–54.
Tian XQ, Chen TC, Matsuoka LY et al. Kinetic and thermodynamic studies of the conversion of previtamin D3 to vitamin D3 in human skin. J Biol Chem 1993; 268: 14888–14892.
Vieth R, Bischoff-Ferrari H, Boucher B et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr 2007; 85: 649–650.
Hollis B . Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr 2005; 135: 317–322.
Holick MF, Binkley NC, Bischoff-Ferrari HA et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2011; 96: 1911–1930.
Hollis BW, Wagner CL . Clinical review: the role of the parent compound vitamin D with respect to metabolism and function: why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab 2013; 98: 4619–4628.
Bikle DD, Gee E, Halloran B et al. Free 1,25-dihydroxyvitamin D levels in serum from normal subjects, pregnant subjects, and subjects with liver disease. J Clin Invest 1984; 74: 1966–1971.
Kumar R, Cohen WR, Silva P et al. Elevated 1,25-dihydroxyvitamin D plasma levels in normal human pregnancy and lactation. J Clin Invest 1979; 63: 342–344.
Lund B, Selnes A . Plasma 1,25-dihydroxyvitamin D levels in pregnancy and lactation. Acta Endocrinol 1979; 92: 330–335.
Steichen JJ, Tsang RC, Gratton TL et al. Vitamin D homeostasis in the perinatal period: 1,25-dihydroxyvitamin D in maternal, cord, and neonatal blood. N Engl J Med 1980; 302: 315–319.
Seino Y, Ishida M, Yamaoka K et al. Serum calcium regulating hormones in the perinatal period. Calcif Tissue Int 1982; 34: 131–135.
Heaney RP, Armas LA, Shary JR et al. 25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am J Clin Nutr 2008; 87: 1738–1742.
Hollis BW, Johnson D, Hulsey TC et al. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res 2011; 26: 2341–2357.
Walker VP, Zhang X, Rastegar I et al. Cord blood vitamin D status impacts innate immune responses. J Clin Endocrinol Metab 2011; 96: 1835–1843.
Eichholzer M, Platz EA, Bienstock JL et al. Racial variation in vitamin D cord blood concentration in white and black male neonates. Cancer Causes Control 2013; 24: 91–98.
Kovacs CS . The role of vitamin D in pregnancy and lactation: insights from animal models and clinical studies. Annu Rev Nutr 2012; 32: 97–123.
Carneiro RM, Prebehalla L, Tedesco MB et al. Lactation and bone turnover: a conundrum of marked bone loss in the setting of coupled bone turnover. J Clin Endocrinol Metab 2010; 95: 1767–1776.
Bell NH, Stern PH, Pantzer E et al. Evidence that increased circulating 1 alpha, 25-dihydroxyvitamin D is the probable cause for abnormal calcium metabolism in sarcoidosis. J Clin Invest 1979; 64: 218–225.
Holick MF . The cutaneous photosynthesis of previtamin D3: a unique photoendocrine system. J Invest Dermatol 1981; 77: 51–58.
Hollander D, Muralidhara KS, Zimmerman A . Vitamin D3 intestinal absorption in vivo: influence of fatty acids, bile salts, and perfusate pH on absorption. Gut 1978; 19: 267–272.
Hollis BW, Lowery JW, Pittard WB 3rd et al. Effect of age on the intestinal absorption of vitamin D3-palmitate and nonesterified vitamin D2 in the term human infant. J Clin Endocrinol Metab 1996; 81: 1385–1388.
Heubi JE, Hollis BW, Specker B et al. Bone disease in chronic childhood cholestasis. I. Vitamin D absorption and metabolism. Hepatology 1989; 9: 258–264.
Haddad JG, Matsuoka LY, Hollis BW et al. Human plasma transport of vitamin D after its endogenous synthesis. J Clin Invest 1993; 91: 2552–2555.
Ponchon G, Kennan AL, DeLuca HF . "Activation" of vitamin D by the liver. J Clin Invest 1969; 48: 2032–2037.
Flanagan JN, Young MV, Persons KS et al. Vitamin D metabolism in human prostate cells: implications for prostate cancer chemoprevention by vitamin D. Anticancer Res 2006; 26: 2567–2572.
Karlgren M, Miura S, Ingelman-Sundberg M . Novel extrahepatic cytochrome P450s. Toxicol Appl Pharmacol 2005; 207: 57–61.
Hosseinpour F, Wikvall K . Porcine microsomal vitamin D(3) 25-hydroxylase (CYP2D25). Catalytic properties, tissue distribution, and comparison with human CYP2D6. J Biol Chem 2000; 275: 34650–34655.
Schuessler M, Astecker N, Herzig G et al. Skin is an autonomous organ in synthesis, two-step activation and degradation of vitamin D(3): CYP27 in epidermis completes the set of essential vitamin D(3)-hydroxylases. Steroids 2001; 66: 399–408.
Zhu J, DeLuca HF . Vitamin D 25-hydroxylase—four decades of searching, are we there yet? Arch Biochem Biophys 2012; 523: 30–36.
Smith JE, Goodman DS . The turnover and transport of vitamin D and of a polar metabolite with the properties of 25-hydroxycholecalciferol in human plasma. J Clin Invest 1971; 50: 2159–2167.
Bouillon R, van Baelen H, de Moor P . Comparative study of the affinity of the serum vitamin D-binding protein. J Steroid Biochem 1980; 13: 1029–1034.
Hollis BW . Comparison of equilibrium and disequilibrium assay conditions for ergocalceferol, cholecaliferol and their major metabolites. J Steroid Biochem 1984; 21: 81–86.
Kissmeyer A, Mathiasen IS, Latini S et al. Pharmacokinetic studies of vitamin D analogues: relationship to vitamin D binding protein (DBP). Endocrine 1995; 3: 263–266.
Vieth R, Kessler MJ, Pritzker KP . Species differences in the binding kinetics of 25-hydroxyvitamin D3 to vitamin D binding protein. Can J Physiol Pharmacol 1990; 68: 1368–1371.
Haddad JG, Hillman L, Rojanasathit S . Human serum binding capacity and affinity for 25-hydroxyergocalciferol and 25-hydroxycholecalciferol. J Clin Endocrinol Metab 1976; 43: 86–91.
Marzolo MP, Farfan P . New insights into the roles of megalin/LRP2 and the regulation of its functional expression. Biol Res 2011; 44: 89–105.
Nykjaer A, Dragun D, Walther D et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 1999; 96: 507–515.
Rasmussen H, Wong M, Bikle D et al. Hormonal control of the renal conversion of 25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol. J Clin Invest 1972; 51: 2502–2504.
Akour AA, Gerk P, Kennedy MJ . Megalin expression in human term and preterm placental villous tissues: effect of gestational age and sample processing and storage time. J Pharmacol Toxicol Methods 2015; 71: 147–154.
Jones AR, Shusta EV . Blood-brain barrier transport of therapeutics via receptor-mediation. Pharm Res 2007; 24: 1759–1771.
Safadi FF, Thornton P, Magiera H et al. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J Clin Invest 1999; 103: 239.
Zella LA, Shevde NK, Hollis BW et al. Vitamin D-binding protein influences total circulating levels of 1,25-dihydroxyvitamin D3 but does not directly modulate the bioactive levels of the hormone in vivo . Endocrinology 2008; 149: 3656–3667.
Bodnar LM, Simhan HN, Catov JM et al. Maternal vitamin D status and the risk of mild and severe preeclampsia. Epidemiology 2014; 25: 207–214.
Robinson CJ, Alanis MC, Wagner CL et al. Plasma 25-hydroxyvitamin D levels in early-onset severe preeclampsia. Am J Obstet Gynecol 2010; 203: 366.e1–e6.
Liu NQ, Ouyang Y, Bulut Y et al. Dietary vitamin D restriction in pregnant female mice is associated with maternal hypertension and altered placental and fetal development. Endocrinology 2013; 154: 2270–2280.
Faulkner JL, Cornelius DC, Amaral LM et al. Vitamin D supplementation improves pathophysiology in a rat model of preeclampsia. Am J Physiol Regul Integr Comp Physiol 2016; 310: R346–R354.
Lykkedegn S, Sorensen GL, Beck-Nielsen SS et al. Vitamin D depletion in pregnancy decreases survival time, oxygen saturation, lung weight and body weight in preterm rat offspring. PLoS One 2016; 11: e0155203.
Calton EK, Keane KN, Newsholme P et al. The impact of vitamin D levels on inflammatory status: a systematic review of immune cell studies. PloS One 2015; 10: e0141770.
McGrath JJ, Eyles DW, Pedersen CB et al. Neonatal vitamin D status and risk of schizophrenia: a population-based case-control study. Arch Gen Psychiatry 2010; 67: 889–894.
LaMarca B, Amaral LM, Harmon AC et al. Placental ischemia and resultant phenotype in animal models of preeclampsia. Curr Hypertens Rep 2016; 18: 38.
Gibson CC, Davis CT, Zhu W et al. Dietary vitamin D and its metabolites non-genomically stabilize the endothelium. PLoS One 2015; 10: e0140370.
Gibson CC, Zhu W, Davis CT et al. Strategy for identifying repurposed drugs for the treatment of cerebral cavernous malformation. Circulation 2015; 131: 289–299.
Black JA, Carter RE . Association between aortic stenosis and facies of severe infantile hypercalcemia. Lancet 1963; 2: 745–749.
Garcia RE, Friedman WF, Kaback M et al. Idiopathic hypercalcemia and supravalvular aortic stenosis: documentation of a new syndrome. N Engl J Med 1964; 271: 117–120.
Friedman WF . Vitamin D as a cause of the supravalvular aortic stenosis syndrome. Am Heart J 1967; 73: 718–720.
Antia AV, Wiltse HE, Rowe RD et al. Pathogenesis of the supravalvular aortic stenosis syndrome. J Pediatr 1967; 71: 431–441.
Seelig MS . Vitamin D and cardiovascular, renal and brain damage in infancy and childhood. Ann N Y Acad Sci 1969; 147: 539–582.
Latorre G . Effect of overdose of vitamin D2 on pregnancy in the rat. Fertil Steril 1961; 12: 343–345.
Friedman WF, Roberts WC . Vitamin D and the supravalvular aortic stenosis syndrome. The transplacental effects of vitamin D on the aorta of the rabbit. Circulation 1966; 34: 77–86.
Morris CA, Mervis CB . William's syndrome and related disorders. Annu Rev Genomics Hum Genet 2000; 1: 461–484.
Aravena T, Castillo S, Carrasco X et al. Williams syndrome: clinical, cytogenetical, neurophysiological and neuroanatomic study. Rev Med Chil 2002; 130: 631–637.
Garabédian M, Jacqz E, Guillozo H et al. Elevated plasma 1,25-dihydroxyvitamin D concentrations in infants with hypercalcemia and an elfin facies. N Engl J Med 1985; 312: 948–952.
Becroft DM, Chambers D . Supravalvular aortic stenosis-infantile hypercalcemia syndrome: in vitro hypersensitivitiy to vitamin D and calcium. J Med Genet 1976; 13: 223–228.
Taylor AB, Stern PH, Bell NH . Abnormal regulation of circulating 25-hydroxyvitamin D on the Williams Syndrome. N Engl J Med 1982; 306: 972–975.
Cooper C, Harvey NC, Bishop NJ et al. Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): a multicentre, double-blind, randomised placebo-controlled trial. Lancet Diabetes Endocrinol 2016; 4: 393–402.
Olsen SF, Secher NJ . A possible preventive effect of low-dose fish oil on early delivery and pre-eclampsia: indications from a 50-year-old controlled trial. Br J Nutr 1990; 64: 599–609.
Bodnar LM, Catov JM, Simhan HN et al. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endorcrinol Metab 2007; 92: 3517–3522.
Baker AM, Haeri S, Camargo CA Jr et al. A nested case-control study of midgestation vitamin D deficiency and risk of severe preeclampsia. J Clin Endocrinol Metab 2010; 95: 5105–5109.
Gernand AD, Bodnar LM, Klebanoff MA et al. Maternal serum 25-hydroxyvitamin D and placental vascular pathology in a multicenter US cohort. Am J Clin Nutr 2013; 98: 383–388.
Merewood A, Mehta SD, Chen TC et al. Association between vitamin D deficiency and primary cesarean section. J Clin Endocrinol Metab 2009; 94: 940–945.
Zhang C, Qiu C, Hu FB et al. Maternal plasma 25-hydroxyvitamin D concentrations and the risk for gestational diabetes mellitus. PLoS One 2008; 3: e3753.
Bodnar LM, Simhan HN . Vitamin D may be a link to black-white disparities in adverse birth outcomes. Obstet Gynecol Surv 2010; 65: 273–284.
Pal L, Zhang H, Williams J et al. Vitamin D status relates to reproductive outcome in women with polycystic ovary syndrome: secondary analysis of a multicenter randomized controlled trial. J Clin Endocrinol Metab 2016; 101: 3027–3035.
Miliku K, Vinkhuyzen A, Blanken LM et al. Maternal vitamin D concentrations during pregnancy, fetal growth patterns, and risks of adverse birth outcomes. Am J Clin Nutr 2016; 103: 1514–1522.
Hou W, Yan XT, Bai CM et al. Decreased serum vitamin D levels in early spontaneous pregnancy loss. Eur J Clin Nutr 2016; 70: 1004–1008.
Lindqvist PG, Silva AT, Gustafsson SA et al. Maternal vitamin D deficiency and fetal distress/birth asphyxia: a population-based nested case-control study. BMJ Open 2016; 6: e009733.
Kiely ME, Zhang JY, Kinsella M et al. Vitamin D status is associated with uteroplacental dysfunction indicated by pre-eclampsia and small-for-gestational-age birth in a large prospective pregnancy cohort in Ireland with low vitamin D status. Am J Clin Nutr 2016; 104: 354–361.
Qin LL, Lu FG, Yang SH et al. Does maternal vitamin D deficiency increase the risk of preterm birth: a meta-analysis of observational studies. Nutrients 2016; 8. pii: E301.
Hollis B, Wagner C . Assessment of dietary vitamin D requirements during pregnancy and Lactation. Am J Clin Nutr 2004; 79: 717–726.
Bodnar LM, Catov JM, Roberts JM . Racial/ethnic differences in the monthly variation of preeclampsia incidence. Am J Obstet Gynecol 2007; 196: 324.e1–e5.
Weiss S, Wolsk H, Mirzakhani H et al.Asthma/wheeze and preeclampsia outcomes in the VDAART trial: the influcence of baseline and treatment levels of vitamin D on treatment response. Boston: The Vitamin D Workshop, 2016. Available at: http://www.vitamindworkshop.org/
Litonjua AA, Carey VJ, Laranjo N et al. Effect of prenatal supplementation with vitamin D on asthma or recurrent wheezing in offspring by age 3 years: The VDAART Randomized Clinical Trial. JAMA 2016; 315: 362–370.
Mirzakhani H, Harshfield B, Carey V et al.(eds). Association of maternal asthma and early pregnancy serum vitamin D level with risk of preeclampsia. Boston: The Vitamin D Workshop. 2016. Available at http://www.vitamindworkshop.org/
Wolsk H, Harshfield BJ, Laranjo N et al. (eds). Vitamin D supplementation in pregnant women of different races and the risk of asthma/recurrent wheeze in the child: findings from the Vitamin D Antenatal Asthma Reduction Trial (VDAART). Boston: The Vitamin D Workshop. 2016. Available at http://www.vitamindworkshop.org/
Wolsk H, Litonjua A, Chawes B et al. Prenatal vitamin D supplementation and risk of asthma/wheeze at 3 years of age: a meta-analysis of randomized controlled trials. JAMA 2016; 315: 353–361.
Wagner CL, McNeil R, Hamilton SA et al. A randomized trial of vitamin D supplementation in 2 community health center networks in South Carolina. Am J Obstet Gynecol 2013; 208: 137.e1–e13.
Wagner CL, Baggerly C, McDonnell S et al. Post-hoc analysis of vitamin D status and reduced risk of preterm birth in two vitamin D pregnancy cohorts compared with South Carolina March of Dimes 2009-2011 rates. J Steroid Biochem Mol Biol 2016; 155: 245–251.
Liu PT, Stenger S, Li H et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006; 311: 1770–1773.
Fabri M, Stenger S, Shin DM et al. Vitamin D is required for IFN-{gamma}-mediated antimicrobial activity of human macrophages. Sci Transl Med 2011; 3: 104ra102.
Liu NQ, Hewison M . Vitamin D, the placenta and pregnancy. Arch Biochem Biophys 2011; 523: 37–47.
Lagishetty V, Liu NQ, Hewison M . Vitamin D metabolism and innate immunity. Mol Cell Endocrinol 2011; 347: 97–105.
Liu NQ, Kaplan AT, Lagishetty V et al. Vitamin D and the regulation of placental inflammation. J Immunol 2011; 186: 5968–5974.
Vieth R . Vitamin D supplementation, 25-hydroxy-vitamin D concentrations, and safety. Am J Clin Nutr 1999; 69: 842–856.
Heaney R, Davies K, Chen T et al. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 2003; 77: 204–210.
Souberbielle JC, Cormier C, Kindermans C et al. Vitamin D status and redefining serum parathyroid hormone reference range in the elderly. J Clin Endocrinol Metab 2001; 86: 3086–3090.
Hollis BW, Wagner CL . Vitamin d and pregnancy: skeletal effects, nonskeletal effects, and birth outcomes. Calcif Tissue Int 2013; 92: 128–139.
Wagner CL, McNeil RB, Johnson DD et al. Health characteristics and outcomes of two randomized vitamin D supplementation trials during pregnancy: a combined analysis. J Steroid Biochem Mol Biol 2013; 136: 313–320.
Goldring ST, Griffiths CJ, Martineau AR et al. Prenatal vitamin d supplementation and child respiratory health: a randomised controlledtrial. PLoS One 2013; 8: e66627.
Sablok A, Batra A, Thariani K et al. Supplementation of vitamin D in pregnancy and its correlation with feto-maternal outcome. Clin Endocrinol (Oxf) 2015; 83: 536–541.
Mojibian M, Soheilykhah S, Fallah Zadeh MA et al. The effects of vitamin D supplementation on maternal and neonatal outcome: a randomized clinical trial. Iran J Reprod Med 2015; 13: 687–696.
Chawes BL, Bonnelykke K, Stokholm J et al. Effect of vitamin D3 supplementation during pregnancy on risk of persistent wheeze in the offspring: a randomized clinical trial. JAMA 2016; 315: 353–361.
Grant CC, Crane J, Mitchell EA et al. Vitamin D supplementation during pregnancy and infancy reduces aeroallergen sensitization: a randomized controlled trial. Allergy 2016; 71: 1325–1334.
Zhang Q, Cheng Y, He M et al. Effect of various doses of vitamin D supplementation on pregnant women with gestational diabetes mellitus: a randomized controlled trial. Exp Ther Med 2016; 12: 1889–1895.
Zerofsky MS, Jacoby BN, Pedersen TL et al. Daily cholecalciferol supplementation during pregnancy alters markers of regulatory immunity, inflammation, and clinical outcomes in a randomized controlled trial.J Nutr 2016; 146: 2388–2397.
Brehm JM, Celedon JC, Soto-Quiros ME et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med 2009; 179: 765–771.
Brehm JM, Schuemann B, Fuhlbrigge AL et al. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol 2010; 126: 52–8 e5.
von Mutius E, Martinez FD . Inconclusive results of randomized trials of prenatal vitamin D for asthma prevention in offspring: curbing the enthusiasm. JAMA 2016; 315: 347–348.
Oxford Centre for Evidence-based Medicine-Levels of Evidence 2009. Available at http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/.
Zhu H, Wagner C, Pan Y et al.Maternal vitamin D supplementation and cord blood genome-wide DNA methylation analysis. (abstract). Boston: Vitamin D Workshop, 2016. Available at http://www.vitamindworkshop.org/
Schulz EV, Cruze L, Wei W et al. Maternal vitamin D sufficiency and reduced placental gene expression in angiogenic biomarkers related to comorbidities of pregnancy. J Steroid Biochem Mol Biol 2017. pii: S0960-0760(17)30038-9.
Al-Garawi A, Carey VJ, Chhabra D et al. The role of vitamin D in the transcriptional program of human pregnancy. PLoS One 2016; 11: e0163832.
Mirzakhani H, Litonjua A, Sharma A et al.Higher Vitamin D Levels in Early Pregnancy and Risk of Preeclampsia. Boston: The Vitamin D Workshop, 2016. Available at http://www.vitamindworkshop.org/
Kiely M, Zhang J, Kinsella M et al.Vitamin D Status is Associated with Utero-placental Dysfunction in a Large Prospective Pregnancy Cohort with Low 25(OH)D3 and Ubiquitous 3-epi-25(OH)D3 and 25(OH)D3 and 25(OH)D2. Boston: The Vitamin D Workshop, 2016, Available at http://www.vitamindworkshop.org/
Anderson CM, Ralph JL, Johnson L et al. First trimester vitamin D status and placental epigenomics in preeclampsia among Northern Plains primiparas. Life Sci 2015; 129: 10–15.
Strid H, Care A, Jansson T et al. Parathyroid hormone-related peptide (38-94) amide stimulates ATP-dependent calcium transport in the Basal plasma membrane of the human syncytiotrophoblast. J Endocrinol 2002; 175: 517–524.
Deftos LJ, Burton DW, Brandt DW et al. Neoplastic hormone-producing cells of the placenta produce and secrete parathyroid hormone-related protein. Studies by immunohistology, immunoassay, and polymerase chain reaction. Lab Invest 1994; 71: 847–852.
Mokry LE, Ross S, Ahmad OS et al. Vitamin D and risk of multiple sclerosis: a Mendelian randomization study. PLoS Med 2015; 12: e1001866.
Whitehouse AJ, Holt BJ, Serralha M et al. Maternal serum vitamin d levels during pregnancy and offspring neurocognitive development. Pediatrics 2012; 129: 485–493.
Chen J, Xin K, Wei J et al. Lower maternal serum 25(OH) D in first trimester associated with higher autism risk in Chinese offspring. J Psychosom Res 2016; 89: 98–101.
Gould JF, Anderson AJ, Yelland LN et al. Association of cord blood vitamin D with early childhood growth and neurodevelopment. J Paediatr Child Health 2017; 53: 75–83.
Patrick RP, Ames BN . Vitamin D hormone regulates serotonin synthesis. Part 1: relevance for autism. FASEB J 2014; 28: 2398–2413.
Patrick RP, Ames BN . Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J 2015; 29: 2207–2222.
Acknowledgements
Funded in part by NIH/NICHD R01 HD043921, the Thrasher Research Fund, NIH/NCATS UL1 RR029882 and UL1 TR000062.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Hollis, B., Wagner, C. New insights into the vitamin D requirements during pregnancy. Bone Res 5, 17030 (2017). https://doi.org/10.1038/boneres.2017.30
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/boneres.2017.30
This article is cited by
-
The impact of vitamin D changes during pregnancy on the development of maternal adverse events: a random forest analysis
BMC Pregnancy and Childbirth (2024)
-
Comparison of cord blood and 6‐month‐old vitamin D levels of healthy term infants supplemented with 400 IU/day dose of vitamin D
European Journal of Clinical Nutrition (2023)
-
“You are my sunshine, my only sunshine”: maternal vitamin D status and supplementation in pregnancy and their effect on neonatal and childhood outcomes
Hormones (2023)
-
Maternal pre-pregnancy BMI and offspring hyperactivity–inattention trajectories from 3 to 8 years in the EDEN birth cohort study
European Child & Adolescent Psychiatry (2023)
-
Knowledge, attitude, performance, and determinant factors of Vitamin D deficiency prevention behaviours among Iranian pregnant women
Archives of Public Health (2021)